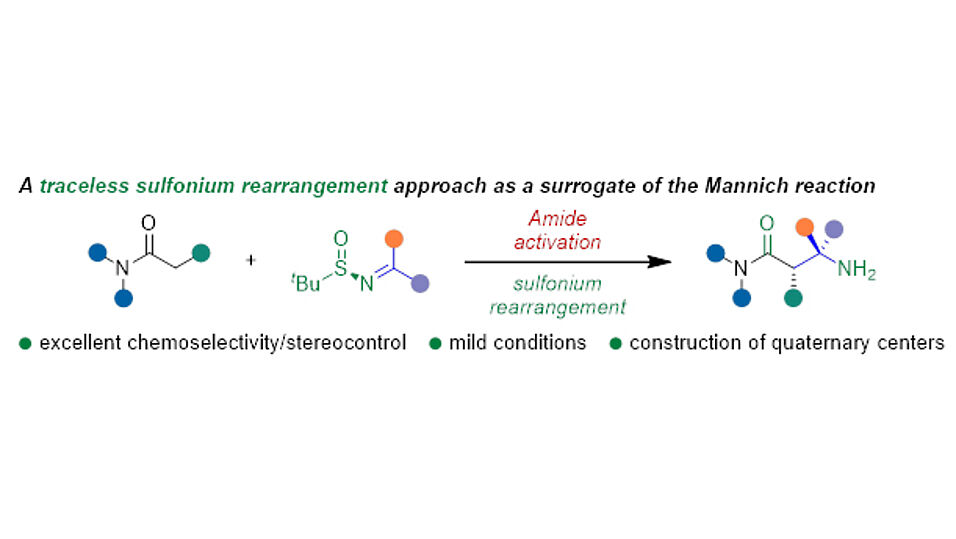
The new method, recently published in the Journal of the American Chemical Society, relies on the combination of two trademarks of the Maulide group: electrophilic amide activation and sulfonium rearrangements. While electrophilic amide activation enables a characteristic and unique chemoselectivity, the integration of enantiopure sulfinimines ensures that this reaction proceeds with high levels of enantioselectivity.
Computational studies, devoted to elucidating the reaction mechanism, uncovered a further remarkable feature of this transformation. While chemical intuition will often base its assessment of likely conformation and configuration on estimates of perceived steric hindrance, dissection of the contributing factors found attractive London dispersion forces to tip the scales in favour of the sterically more congested diastereomer.
Publication in JACS:
Deployment of Sulfinimines in Charge-Accelerated Sulfonium Rearrangement Enables a Surrogate Asymmetric Mannich Reaction; Minghao Feng, Ivan Mosiagin, Daniel Kaiser, Boris Maryasin and Nuno Maulide, J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c05368