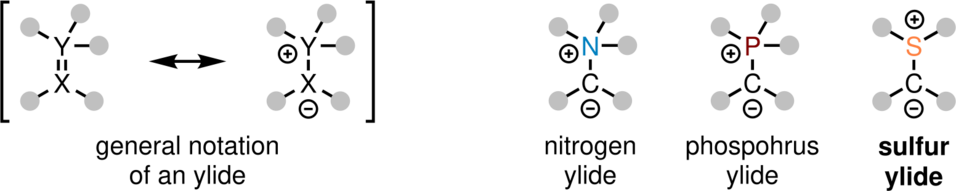
An ylide is a neutral chemical compound containing a formally negatively charged atom (e.g. carbon) directly bound to a formally positively charged one, such as nitrogen, phosphorus or sulfur.
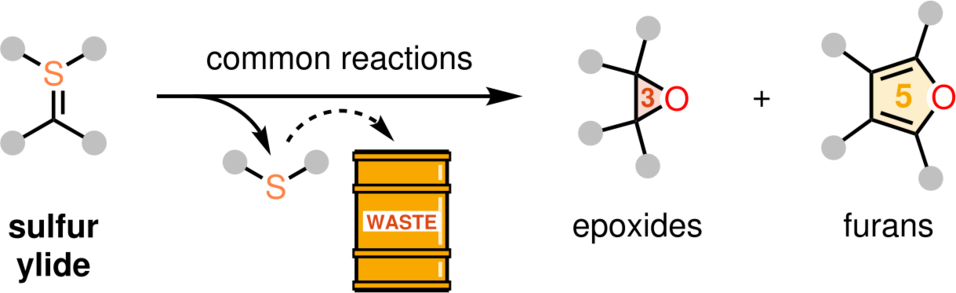
Since the middle of the last century, sulfur ylides have evolved as textbook organic reagents in multiple transformations. Particularly, these motifs have been exploited for the synthesis of 3-membered (e.g. epoxides) or 5-membered rings (e.g. furans).
Avoiding waste
However, most of these reactions are accompanied by a crucial disadvantage: the release of the sulfur moiety, leading to an increased amount of waste.
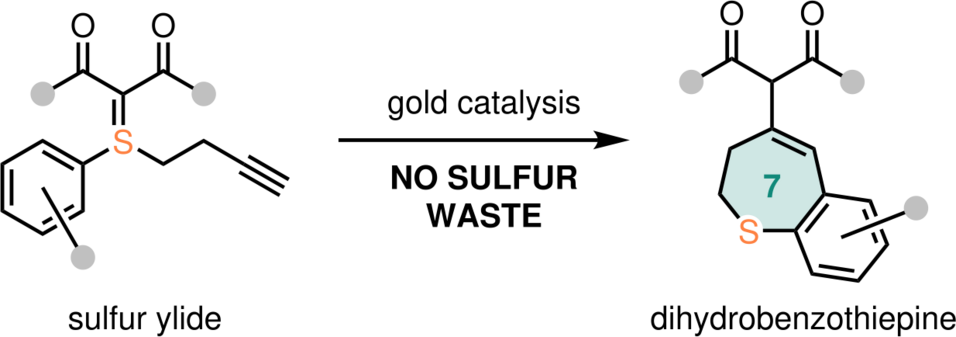
By developing a reaction of sulfur ylides that proceeds without the generation of this waste, Nuno Maulide from the Faculty of Chemistry and his team have disclosed an attractive and economical addition to ylide chemistry. The incorporation of the sulfur atom into the final product, thereby trapping it, results in the formation of highly functionalised sulfur-containing substances.
More precisely, this study developed the synthesis of 7-membered sulfur-based rings attached to benzene, structures known as dihydrobenzothiepines. This "atom-economical protocol" according to the authors takes place in the presence of a gold catalyst, "under very mild conditions and at ambient temperature", affording a large number of novel dihydrobenzothiepine derivatives.
S E R V I C E: Gold-catalyzed cycloisomerization of sulfur ylides to dihydrobenzothiepines. Christian Knittl-Frank, Iakovos Saridakis, Thomas Stephens, Rafael Gomes, James Neuhaus, Antonio Misale, Rik Oost, Alberto Oppedisano and Nuno Maulide, in: Chemistry – A European Journal, Chem. Eur. J. 10.1002/chem.202000622
Maulide Group: https://organicsynthesis.univie.ac.at/