
The Faculty Colloquium usually takes place every second Monday each month during the semester (four talks). National and international scientists as well as scientists from our Faculty of Chemistry give insight into their field of research in the framework of 45-minute lectures.
Before the colloquia, there is a faculty coffee, during which faculty members and students are cordially invited to meet for an exchange over coffee and cake.
Program Coordination: Univ.-Prof. Dr. Nuno Maulide
ECTS
If at least three of the four lectures have been attended, this course will have a credit of 0.5 ECTS.

Monday, 24 March 2025, 16:00 / Joseph Loschmidt lecture hall (LH 2) of the Faculty of Chemistry:
Dr. Charlotte Gers-Panther, Editor, Angewandte Chemie at Wiley-VCH
„Scientific Publishing – A Peek into the Editorial Office and Beyond”.
Have you ever wondered:
- How to pick the right journal for your manuscript?
- How to best prepare your manuscript for submission?
- How the peer review process works?
- What the benefits of Open Access publishing are?
These questions, and many more, will be answered in the lecture „Scientific Publishing – A Peek into the Editorial Office and Beyond”.

Monday, 07 April 2025, 16:00 / Joseph Loschmidt lecture hall (LH 2) of the Faculty of Chemistry:
Univ. Prof. Dr. Bernhard LENDL, Vibrational Spectroscopy at TU Vienna / Head of the CAVS Research Group, Austria
"Advances in mid-IR laser spectroscopy: New measurement modalities and applications"
Mid-infrared (Mid-IR) quantum cascade lasers stand out as coherent and polarized sources, offering rapid intensity and wavelength modulation capabilities. These features enable the development of advanced sensing strategies that extend beyond traditional absorption spectroscopy, which relies on the Beer-Lambert law.
When interacting with gaseous or liquid samples, laser radiation does more than attenuate light intensity; it also induces a phase shift in the transmitted light and heats the sample containing the analyte. While direct absorption spectroscopy primarily exploits intensity attenuation, these additional effects form the basis for dispersion spectroscopy and indirect methods like photoacoustic and photothermal spectroscopies.
This presentation will review potential measurement modalities, highlight recent instrumental advancements, and showcase selected applications. For liquid sensing, we employ broadly tunable external cavity quantum cascade lasers. We demonstrate their use in measuring protein secondary structures in water, exemplified by in-line detection in liquid chromatography and protein melting experiments. Dispersion spectroscopy will be introduced through measuring ethanol in water, illustrating its suitability for dynamic reaction monitoring. Finally recent developments in photothermal spectroscopy for trace gas sensing and near-field IR imaging will be introduced as well.

Monday, 12 May 2025, 16:00 / Joseph Loschmidt lecture hall (LH 2) of the Faculty of Chemistry:
Prof. Dr. Martina Havenith-Newen, Department of Physical Chemistry II, Ruhr University Bochum, Germany
"Caught in the act: Time-resolved THz spectroscopy puts the spotlight on the role of the solvent into photochemical reactions"
The development of time-resolved and surface-sensitive spectroscopies in the UV, optical, and IR frequency range have impacted our understanding of chemical reactions. Probing intermediates and transition states can now be probed experimentally and, in doing so, provide new perspectives on reaction pathways and dynamics. These techniques have so far mainly focused on the solutes, the reactants, and the products. Our goal is to put the spotlight on the solvent and visualize the correlated electron and solvent dynamics in electrocatalysis and photochemical reactions. While optical and IR spectroscopy are able to report on the electronic states and the Stokes shift and on the intramolecular modes, they provide less information on non-covalent and intermolecular interactions that are involved. We use THz spectroscopy as a most sensitive tool to probe intermolecular interactions such as hydrogen bonding and local solvation motifs [1,2]. Most notable, changes in these can be directly correlated to changes in solvation entropy and enthalpy, e.g., changes in free energy upon desolvation and resolvation [3]. Unlike conventional calorimetry, “THz calorimetry” which is based on spectroscopic observables, can be applied to inhomogeneous samples and can monitor the solvent response and the propagation of vibrational energy upon photoexcitation on a sec time scale by optical pump terahertz probe (OPTP) spectroscopy. Initial experiments revealed new insights into the solvent dynamics of prototype photoreactions [4,5].
References:
1. S. Pezzotti, W. Chen, F. Novelli, X. Yu, C. Hoberg, M. Havenith, Terahertz-calorimetry spotlights the role of water in biological processes, Nat. Rev. Chem., accepted (2025).
2. K. Mauelshagen, P. Schienbein, I. Kolling, G. Schwaab, D. Marx, M. Havenith, Random encounters dominate water-water interactions at supercritical conditions, Sci. Adv. 11, eadp8614 (2025).
3. S.S. Nalige, P. Galonska, P. Kelich, L. Sistemich, C. Herrmann, L. Vukovic, S. Kruss, M. Havenith, Fluorescence changes in carbon nanotube sensors correlate with THz absorption of hydration, Nat. Commun. 15, 6770 (2024).
4. C. Hoberg, J.J. Talbot, J. Shee, T. Ockelmann, D. Das Mahanta, F. Novelli, M. Head-Gordon, M. Havenith, Caught in the act: Real-time observation of the solvent response that promotes excited-state proton transfer in pyranine, Chem. Sci. 14, 4048–4058 (2023).
5. F. Novelli, K. Chen, A. Buchmann, T. Ockelmann, C. Hoberg, T. Head-Gordon, M. Havenith, The birth and evolution of solvated electrons in the water, PNAS 120, e2216480120 (2023).

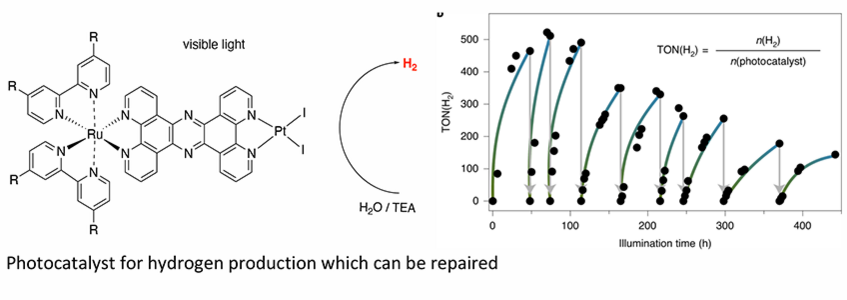
Monday, 02 June 2025, 16:00 / Carl Auer von Welsbach lecture hall (LH1) of the Faculty of Chemistry:
Prof. Dr. Sven Rau, University of Ulm, Institute of Inorganic Chemistry, Germany
"Active repair of molecular hydrogen evolving photocatalysts - a new chapter in artificial photosynthesis"
Photocatalytic water splitting can be utilised with molecular photocatalysts. [1] Ruthenium complexes with related tpphz type ligands play an important role within this field. [2] Through a series of model catalysts we now can differentiate between several important structural aspects influencing the photocatalytic activity.
One very limiting factor for applying molecular catalysts is their propensity to loss of activity. Nature has devised elaborate repair systems restoring catalytic activity of biological molecular defined catalysts. [3-4] We present here a new concept on repairing the below described molecular photocatalyst and thereby boosting catalytic performance.
References:
[1] Proton-Coupled Electron Transfer: The Engine of Energy Conversion and Storage D. G. Nocera, J. Am. Chem. Soc. 2022, 144, 1069−1081
[2] M. G. Pfeffer, C. Müller, E. T. E. Kastl, A. K. Mengele, B. Bagemihl, S. S. Fauth, J. Habermehl, L.
Petermann, M. Wächtler, M. Schulz, D. Chartrand, F. Laverdière, P. Seeber, S. Kupfer, S. Gräfe, G.
S. Hanan, J. G. Vos, B. Dietzek-Ivanšić, S. Rau, Nat. Chem., 2022, 14, 500-506
[3] Learning from Nature’s Example: Repair Strategies in Light-Driven Catalysis A. K. Mengele, S.
Rau, JACS Au, 2023, 3, 36-46
[4] Rationalizing In Situ Active Repair in Hydrogen Evolution Photocatalysis via Non-Invasive Raman
Spectroscopy S. Klingler, B. Bagemihl, A. K. Mengele, S. Kaufhold, P. Myllyperkiö, J. Ahokas, M.
Pettersson, S. Rau, B. Mizaikoff, Angew. Chem. Int. Ed. 2023, e202306287 DOI:
org/10.1002/anie.202306287
