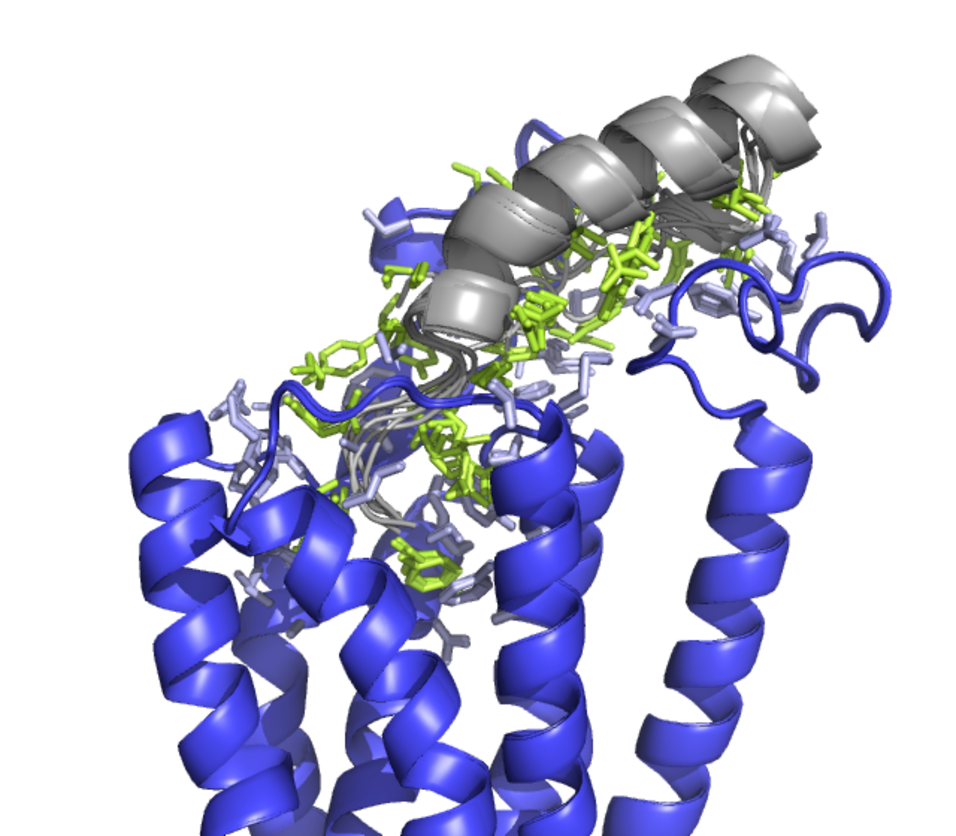
Peptides hormones play an important role in the regulation of manifold activities in the body. Many of them transmit their activity through G-protein coupled receptors (GPCR), which are among the most promising drug targets nowadays. Accordingly, elucidating the binding mode of ligands is essential. Whereas several small molecule systems have been well characterized, ligand binding of large and flexible peptides is still more challenging. In addition to ligand binding and receptor activation, indirect mechanisms have been shown to play a role for drugs addressing GPCRs. This includes desensitation, internalisation and accordingly their potential use as drug shuttles, e. g. in tumour targeting. Accordingly, in addition to ligand binding, internalization has to be addressed and to be studied, including arrestin recruitment. Accordingly, ligand binding, structural dynamics and internalization has to be addressed and to be studied to address G protein coupled receptors for drug development.
The neuropeptide Y/pancreatic polypeptide family contains 36 amino acid peptides that bind in human to four different so-called Y-receptors. By a combination of X-ray analysis, NMR, molecular modelling and crosslinking combined with mass spectrometry, we could recently identify the distinct binding modes of NPY to the Y1- and the Y2 receptors [1,2]. We could further demonstrate that chemical modification of the ligand, including fluorescence labelling, lipidisation and PEGylation significantly modifies the trafficking of the ligand [3]. By labelling of the receptor with a novel template-assisted ligation strategy [4], we can follow ligand/receptor complexes in living cells. Furthermore, we identified a different mode of arrestin binding and recruitment [5]. Neuropeptide Y1 and Y2 receptors have been shown to play a relevant role in different tumours. In breast cancer we demonstrated that human Y1 receptors are addressable by peptide conjugates using 99mTc or 18F PET-tracers [6,7]. We now designed Y1 receptor selective peptides linked to different toxophors [8,9]. Furthermore, we characterized the mechanism of direct and peptide-mediated uptake of tubulysin-related toxins [8]. In the field of tumour therapy, peptide-drug conjugates are already well accepted. However, the concept of receptor-mediated internalisation and subsequent tissue specific intracellular application is not limited to the selective addressing of tumours. This may open up a new field of targeted therapy by mid-sized drugs.
____
[1] Kaiser A et al. Angew Chem Int Ed Engl.2015, 54, 7446-7449.
[2] Yang Z, Han S, Keller M, Kaiser A, Bender B et al., Nature 2018, 556, 520-524.
[3] Mäde V et al. Angew Chem Int Ed Engl. 2014,53, 10067-10071.
[4] Reinhardt U et al. Angew Chem Int Ed Engl. 2014,53, 10237-10241.
[5] Wanka L et al. Cell Signal. 2018, 50,58-71.
[6]Khan IU et al. Angew Chem Int Ed Engl. 2010, 49, 1155-8.
[7] Hofmann S et al. Mol Pharm. 2015, 12, 1121-30.
[8] Ahrens VM et al. J. Contr. Release 2015, 209,170-8.
[9] Böhme D, Krieghoff J, Beck-Sickinger AG. J Med Chem. 2016 Apr 14;59, 3409-17.
Prof. Dr. Annette Beck-Sickinger is Full Professor (C4) of Bioorganic Chemistry and Biochemistry of the Institute of Biochemistry, Faculty of Life Sciences, Leipzig University. Her main research topics are: a) Protein-protein interaction, especially interaction of peptide/protein hormones and G protein-coupled receptors; b) Therapeutic peptides/proteins, development, characterization and application; and c) Peptide/protein modification in biomaterials in order to modify surfaces. In all fields, a tight connection of chemical methods, bioorganic synthesis and molecular biology tools, including cloning, receptor mutagenesis, protein expression and cell biochemistry is applied.
https://biochemie.lw.uni-leipzig.de/arbeitsgruppen/biochemie-und-bioorganische-chemie/