The Department of Organic Chemistry kindly invites to the following talk:
Prof. Dr. Rafal Klajn, Institute of Science and Technology Austria (ISTA)
“Controlling chemical reactivity by confinement effects”
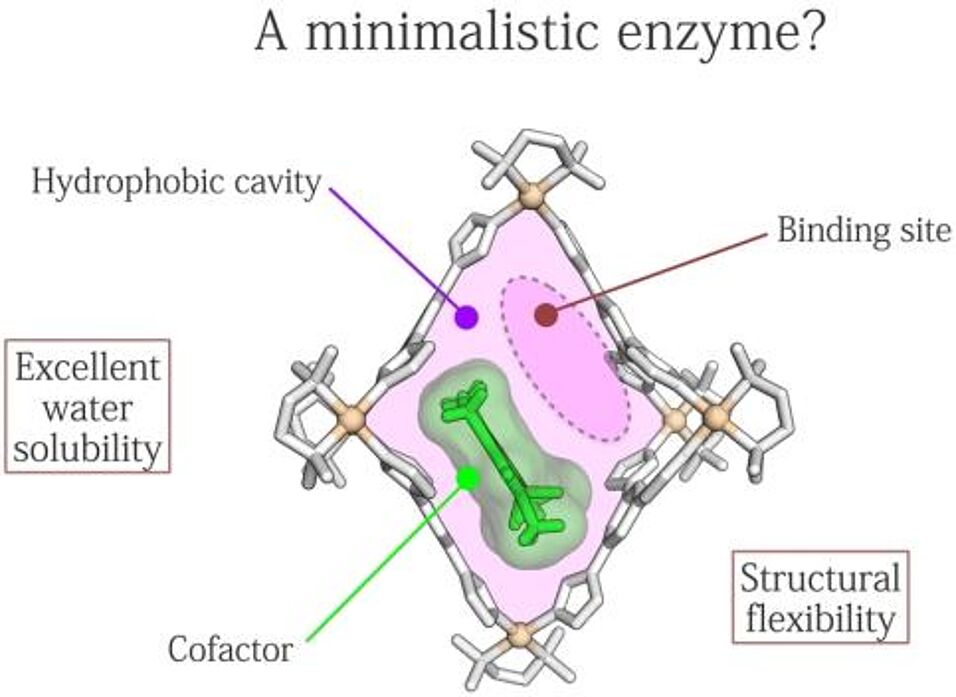
Nature has long impressed chemists with its ability to stabilize ephemeral chemical species, perform chemical reactions with unprecedented rates and selectivities, and synthesize complex organic molecules and exquisiteinorganic structures seemingly effortlessly. What the natural systems consistently exploit is the aspect of confinement. In contrast, the use of confinement effects in a synthetic setup (1) is relatively rare; examples include “on-water”catalysis (2) and reaction acceleration within molecular containers. (3) In this talk, I will discuss our recent studies on the synthesis and properties of molecules and porous nanomaterials capable of confining other, smaller molecules. These nanomaterials include colloidal crystals (4) and nanoporous metals (5) and feature confined spaces with dimensions of 1–10 nm; the cavities of molecular containers are smaller, typically in the subnanometer size regime. (6) I will describehow these confined spaces can be generated reversibly using light (7) and magnetic fields, (8) paving the way towardcontrolling chemical reactivity using external stimuli. Finally, I will focus on the cavities of coordination cages (9) anddiscuss how we utilized them to tune the wavelength of azobenzene photoisomerization across the entire visiblerange. (10)
1 B. Mitschke, M. Turberg, B. List, Confinement as a unifying element in selective catalysis, Chem 2020, 6, 2515–2532.
2 A. B. Grommet, M. Feller, R. Klajn, Chemical reactivity under nanoconfinement, Nat. Nanotechnol. 2020, 15, 256–271.
3 S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb, K. B. Sharpless, “On water”: Unique reactivity of organic compoundsin aqueous suspension, Angew. Chem. Int. Ed. 2005, 44, 3275–3279.
4 M. Yoshizawa, J. K. Klosterman, M. Fujita, Functional molecular flasks: New properties and reactions within discrete, selfassembledhosts, Angew. Chem. Int. Ed. 2009, 48, 3418–3438.
5 T. Bian et al., Electrostatic co-assembly of nanoparticles with oppositely charged small molecules into static and dynamicsuperstructures, Nat. Chem. 2021, 13, 940–949.
6 T. Udayabhaskararao et al., Tunable porous nanoallotropes prepared by post-assembly etching of binary nanoparticlesuperlattices, Science 2017, 358, 514–518.
7 H. Zhao et al., Reversible occlusion and reaction acceleration within dynamically self-assembling nanoflasks, Nat. Nanotechnol.2016, 11, 82–88.
8 G. Singh, H. Chan, A. Baskin, E. Gelman, N. Repnin, P. Kral, R. Klajn, Self-assembly of magnetite nanocubes into helicalsuperstructures Science 2014, 345, 1149–1153.
9 D. Samanta et al., Reversible photoswitching of encapsulated azobenzenes in water, Proc. Natl. Acad. Sci. USA 2018, 115,9379–9384.
10 J. Gemen et al., Disequilibrating azobenzenes by visible-light sensitization under confinement, Science 2023, 381, 1357–1363.